DRUG SHORTAGES
HHS Should Implement a Mechanism to Coordinate Its Activities
Report to Congressional Addressees
United States Government Accountability Office
View GAO‑25‑107110. For more information, contact Mary Denigan-Macauley at deniganmacauleym@gao.gov.
Highlights of GAO‑25‑107110, a report to congressional addressees
HHS Should Implement a Mechanism to Coordinate Its Activities
Why GAO Did This Study
Drug shortages arise from a variety of factors that contribute to supply chain vulnerabilities, such as lack of incentives to produce less profitable drugs and to invest in manufacturing quality. While FDA helps respond to and prevent drug shortages, it cannot address some of the economic factors affecting the supply chain like other agencies can, such as by purchasing drugs or funding certain manufacturing.
The CARES Act includes a provision for GAO to report on the federal pandemic response. This report (1) describes the trends in drug shortages since the start of the COVID-19 pandemic, (2) describes steps FDA is taking to improve its drug shortage response and prevention efforts, and (3) examines the status of the Supply Chain Resilience and Shortages Coordinator position.
GAO analyzed FDA data from 2017 to 2024 to obtain information on drug shortages; identified new efforts that FDA had underway since GAO last reported on the issue in 2016; reviewed relevant FDA documents and guidance; and interviewed officials from HHS and a nongeneralizable sample of 15 organizations representing entities affected by drug shortages, such as manufacturers, patients, and providers.
What GAO Recommends
GAO is making two recommendations: that the Secretary of Health and Human Services (1) identify and implement a mechanism to formally coordinate its drug shortage activities and collaborate with other federal stakeholders, and (2) ensure this mechanism takes GAO leading practices for collaboration into consideration.
When HHS was provided a draft of this report for review, GAO recommended that the coordinator document how GAO’s leading practices will be used for coordinating across the federal government to help address drug shortages. In written comments, HHS stated that it did not concur with GAO’s recommendation, as the coordinator position and its associated actions would be ending in May 2025. Consistent with this new information, GAO revised its recommendation, as stated above.
What GAO Found
Drug shortages are a serious public health concern that can adversely affect patients by delaying or limiting access to care. Challenges with the Food and Drug Administration’s (FDA) oversight of medical products, including drug shortages, led to its inclusion on GAO’s High-Risk List. As of July 31, 2024, there were 102 drug shortages being tracked by FDA. Since the start of the COVID-19 pandemic in 2020, the number of new drug shortages reported each year has generally decreased, although drug shortages are lasting longer. The types of drugs in shortage generally continued pre-pandemic trends. For example, shortages most commonly affect sterile injectable drugs that are critical to hospital care and cancer treatment. Further, the pandemic exacerbated existing supply chain vulnerabilities that underlie shortages. For example, shortages of a drug used to prevent blood clotting during surgeries were exacerbated by demand increases during the pandemic. This affected patient care in life-threatening situations, according to a patient advocacy group.
FDA, within the Department of Health and Human Services (HHS), is responsible for tracking and addressing drug shortages in the U.S. As such, FDA has several efforts underway to improve how it addresses shortages. For example, FDA has taken steps to develop data analytic tools to help its staff better analyze drug supply chain information and potentially predict possible drug supply disruptions. FDA also started developing an effort to encourage manufacturers to invest in more mature quality systems, as quality issues underlie many shortages.
Drug shortages are a multifaceted issue that require a collaborative governmental approach to address them, according to FDA, Congress, and others. However, HHS did not have a coordinating structure across the department to oversee its responses and strategies. This limited the capability of HHS to mitigate and respond to shortages and strengthen supply chain resilience, according to HHS. In November 2023, President Biden announced a coordinator position within HHS to strengthen medical product supply chains and address related shortages. HHS took steps to establish this position. For example, it appointed an acting coordinator that developed a task force that included representatives from agencies across HHS.
Given the longstanding nature of this critical public health issue, it is important that HHS identify and implement a mechanism to coordinate its drug shortage activities and collaborate with other federal stakeholders. Once a mechanism is identified, taking into consideration GAO’s leading practices for interagency collaboration when developing that mechanism will be critical to ensuring HHS can effectively address drug shortages.
GAO's Leading Interagency Collaboration Practices and Selected Key Considerations

|
Abbreviations |
|
|
ADHD |
attention-deficit/hyperactivity disorder |
|
ASPE |
Office of the Assistant Secretary for Planning and Evaluation |
|
ASPR |
Administration for Strategic Preparedness and Response |
|
CMS |
Centers for Medicare & Medicaid Services |
|
DOD |
Department of Defense |
|
DEA |
Drug Enforcement Administration |
|
FDA |
Food and Drug Administration |
|
HHS |
Department of Health and Human Services |
|
QMM |
Quality Management Maturity |
|
VA |
Department of Veterans Affairs |
This is a work of the U.S. government and is not subject to copyright protection in the United States. The published product may be reproduced and distributed in its entirety without further permission from GAO. However, because this work may contain copyrighted images or other material, permission from the copyright holder may be necessary if you wish to reproduce this material separately.
April 9, 2025
Congressional Addressees
Drug shortages are a serious public health concern that can adversely affect patients by delaying or limiting access to care. A limited supply of drugs could, for example, require providers to make difficult decisions on which cancer patients should start or complete a round of chemotherapy. A variety of factors can lead to supply chains being vulnerable to disruptions, and drug shortages often result most directly from a problem that limits supply, such as a shutdown caused by a manufacturing interruption, or from an unexpected increase in demand. For example, in 2024, Hurricane Helene caused widespread shortages of intravenous fluids used by patients in hospitals and during dialysis when it damaged a large manufacturer in North Carolina.
The Food and Drug Administration (FDA), within the Department of Health and Human Services (HHS), oversees the safety and effectiveness of drugs marketed in the U.S. In this capacity, it is responsible for helping to address—prevent, mitigate, and resolve—drug shortages. Manufacturers of certain drugs are required to notify FDA in advance of a potential drug shortage, and the agency takes actions in response.[1]
We have previously reported that FDA’s efforts to address shortages had shortcomings.[2] For example, in 2014, we found that data challenges hindered FDA’s efforts to understand the causes of specific shortages and undermined its efforts to prevent them from occurring.[3] We also found that FDA had not conducted routine analyses of the shortage data it maintained to proactively identify and evaluate the risks of drug shortages. We recommended that FDA conduct such analyses. FDA agreed with our recommendation. As of December 2024, that recommendation had not been fully implemented. This and other challenges contributed to us including FDA’s oversight of medical products in GAO’s High-Risk List.[4]
There are other HHS agencies and federal departments that play a role in addressing drug shortages. For example, HHS’s Administration for Strategic Preparedness and Response (ASPR) provides funds to support new drug manufacturing to spur supply and the Department of Justice’s Drug Enforcement Administration (DEA) can adjust limits on the manufacture of certain drugs to increase or decrease supply. In November 2023, President Biden announced several initiatives as part of a broader effort to mitigate drug shortages. These included the establishment of a new HHS-designated Supply Chain Resilience and Shortages Coordinator to strengthen medical product supply chains and address related shortages.[5]
While drug shortages are a longstanding issue, the COVID-19 pandemic called attention to supply chain vulnerabilities and concerns about shortages of drugs necessary for the response. Based partly on FDA and other HHS agencies’ response to the pandemic, we added HHS leadership and coordination of public health emergencies to our High-Risk List in 2022.[6] The CARES Act includes a provision for us to report on the federal response to the COVID-19 pandemic.[7] This report
1. describes the trends in drug shortages that the U.S. has experienced since the start of the COVID-19 pandemic,
2. describes steps FDA is taking to improve its drug shortage response and prevention efforts, and
3. examines the status of the Supply Chain Resilience and Shortages Coordinator position.
To describe trends in drug shortages the U.S. has experienced since the start of the COVID-19 pandemic, we reviewed FDA data and interviewed FDA officials.[8] Specifically, we examined data from FDA’s annual reports, its public facing drug shortage database, and its internal database. We reviewed FDA’s annual drug shortage reports from 2017 (to establish trends prior to the start of COVID-19 pandemic) through 2023 (the most recent published report) to obtain information on and describe trends in new, ongoing, and prevented shortages before, during, and after the COVID-19 pandemic, which began in January 2020.
We also analyzed data from the publicly available FDA Drug Shortage Database to obtain information on the duration of shortages and reported reasons for shortages, among other things. We analyzed data from 2019 (prior to the start of the COVID-19 pandemic), 2021 (1 year after the start of the COVID-19 pandemic), and 2024 (the most recent data available at the time of our review) to examine trends in shortages both before and after the start of the COVID-19 pandemic. The 2024 data were extracted from a live version of the database on July 31, 2024, while 2021 and 2019 data were extracted from archived versions of the database. The archived extracts for those years were available for April 12, 2021, and December 29, 2019. The database is updated daily; therefore, we conducted point-in-time analyses using versions of the database from specific days. For this analysis, we excluded drugs that were discontinued but not actively in shortage. We also analyzed data on resolved shortages from 2019 through 2023 that FDA provided from its internal database.
Additionally, using the 2024 data from the public database and internal data on resolved shortages, we selected a judgmental sample of 10 drugs that were either in shortage or could have potentially gone into shortage, and reviewed narrative information on actions FDA took to address active, resolved, and prevented shortages. We requested these narratives from FDA, as this information was not available through the publicly available database. We selected the sample to represent a diverse range of drugs and their characteristics, including status of shortage, therapeutic category, duration of shortage, and effects on patient care. In addition, we included drug shortages that started before or during the COVID-19 pandemic.
We assessed the reliability of FDA’s data from all three sources by reviewing agency documentation, interviewing agency officials, and conducting manual reviews of the data for errors. Based on these assessments, we determined that these data were sufficiently reliable for the purposes of our report.
To describe steps FDA is taking to improve its drug shortage response and prevention efforts, we identified new efforts that FDA had underway since we previously reported on drug shortages in 2016.[9] To identify these efforts, we reviewed relevant agency documentation and interviewed FDA officials on new and ongoing drug shortage efforts. Additionally, we interviewed FDA officials and a random sample of five of the eight FDA staff responsible for addressing drug shortages to obtain information on work performed to address shortages, including their workload and documentation and workflow processes.
To examine the status of the Supply Chain Resilience and Shortages Coordinator position, we reviewed the White House Fact Sheet and agency documentation describing the position’s scope and responsibilities.[10] We also interviewed HHS officials responsible for developing the Coordinator position to obtain information on the Coordinator’s responsibilities and progress developing the action plan to guide those responsibilities. We assessed the development and planned actions of the Coordinator position against GAO’s Leading Practices to Enhance Interagency Collaboration and Address Crosscutting Challenges.[11]
To further address all three reporting objectives, we interviewed a nongeneralizable sample of officials from 12 organizations representing academic and research institutions, trade associations, associations representing drug manufacturers and pharmaceutical companies, and health care providers.[12] We selected these organizations because they represent drug manufacturers or entities affected by drug shortages. We also interviewed representatives from three patient advocacy groups to obtain information on the effects drug shortages have on patient care. We selected a nongeneralizable sample of groups that represent individuals that may be affected by specific types of drugs in shortage, such as individuals with cancer.[13] We also reviewed drug shortage research conducted by external organizations.
We conducted this performance audit from October 2023 to April 2025 in accordance with generally accepted government auditing standards. Those standards require that we plan and perform the audit to obtain sufficient, appropriate evidence to provide a reasonable basis for our findings and conclusions based on our audit objectives. We believe that the evidence obtained provides a reasonable basis for our findings and conclusions based on our audit objectives.
Background
Causes of Drug Shortages
Under the Federal Food, Drug, and Cosmetic Act, a drug shortage occurs when the demand or projected demand for a drug within the U.S. exceeds the supply of the drug for a period of time.[14] In 2019, an FDA-led, inter-agency Drug Shortages Task Force issued a report analyzing the root causes of drug shortages in response to a congressional request.[15] According to that report, drug shortages persist because the market is not responding as it typically should. In a more typical market, prices rise after a supply disruption, and this provides an incentive for existing and new suppliers to increase production until there is enough supply of a product to meet demand. However, the Task Force report found that the market for prescription drugs differs from other markets and does not respond in this way. The report found that this was especially true for generic drugs, which have the same active ingredients as brand-name drugs but are typically lower cost for consumers and less profitable for manufacturers.
The Task Force’s report identified three major root causes that contribute to these conditions and lead to drug shortages.
1. Lack of incentives to produce less profitable drugs. When market conditions limit manufacturers’ profitability, this reduces a manufacturer’s motivation to maintain a presence in, or enter, the market and to invest in manufacturing quality and redundant capacity.[16]
2. Lack of recognition and reward for manufacturers with mature quality management systems. All manufacturers must meet regulatory requirements from FDA that set a minimum threshold for manufacturing quality. However, the report found there were a lack of incentives for manufacturers to go beyond those minimum thresholds. It also found a lack of publicly available information that would allow drug purchasers to identify manufacturers that go beyond those minimum thresholds and pay higher prices for their drugs. As a result, the report found that manufacturers are more likely to keep costs down by minimizing investments in manufacturing quality, which eventually leads to quality problems, triggering supply disruptions and shortages.
3. Logistical and regulatory challenges. While the supply chain has evolved in a way that makes it more vulnerable to drug shortages by becoming longer, more complex, and more fragmented, the logistical and regulatory structures remained relatively the same. For example, manufacturers face capital, technological, and active pharmaceutical ingredient sourcing challenges that make it difficult to expand production capacity. Additionally, in general, a manufacturer that wants to expand capacity must submit a regulatory filing to have any new supplier or manufacturing facilities approved by FDA.
Federal Role in Preventing and Resolving Drug Shortages
Under federal law, FDA has specific responsibilities related to drug shortages, such as maintaining an up-to-date list of drugs experiencing a shortage (referred to as “in shortage”).[17] FDA determines whether a drug is in shortage using information that manufacturers are required by law to report.[18] Specifically, this reporting includes notifications of any permanent discontinuance or interruption in manufacturing that is likely to lead to a meaningful disruption in supply of the drug and the reason for that discontinuance or interruption. FDA may also receive notifications of actual and potential drug shortages from health professionals and the public.
FDA’s Drug Shortage Staff coordinate the agency’s activities to prevent, alleviate, and resolve drug shortages.[19] When the Drug Shortage Staff receive a notification from a manufacturer of a permanent discontinuance or manufacturing interruption, they take steps to identify whether a shortage of that drug will occur or is occurring. (See fig. 1.) For example, they attempt to determine whether the drug is medically necessary and if the drug is produced by a single manufacturer (sole-source) or more than one manufacturer (multi-source).[20] The Drug Shortage Staff also determine if the total supply of the drug and any drugs that are pharmaceutical equivalents is inadequate to meet demand. To verify that a shortage is in effect, or a potential shortage is pending, the Drug Shortage Staff contacts all manufacturers of the drug to collect up-to-date information on the inventory and demand. If a shortage is determined to exist, the Drug Shortage Staff then include the drug on FDA’s public-facing drug shortage list. This list includes information about the shortage, such as the drug name and reason for the shortage reported by manufacturers. The reasons used for the drug shortage list are set by statute and are: delay in shipping of the drug, demand increase for the drug, discontinuation of the manufacture of the drug, regulatory delay, requirements related to complying with good manufacturing practices, shortages of an active ingredient, or shortages of an inactive ingredient.[21] If none of these statutory reasons apply, FDA allows manufacturers to report the reason for the shortage as “other.”
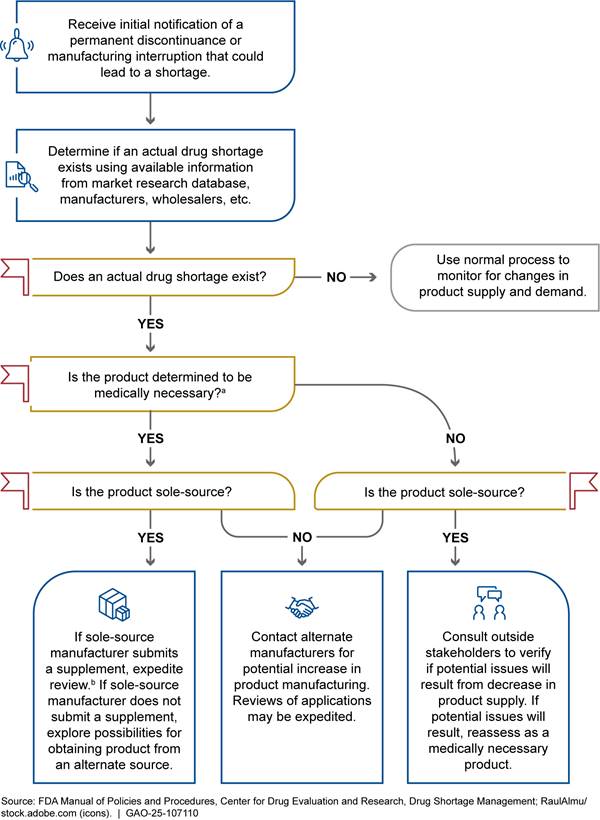
aA medically necessary drug is used to treat or prevent a serious disease or medical condition for which an acceptable alternative drug product or therapy is not available, according to the Food and Drug Administration (FDA).
bA supplement is an application submitted by a manufacturer to notify FDA of a change to its previously approved drug application, such as a new manufacturing site.
Once FDA identifies a drug in shortage, the agency may be able to take a number of actions to help prevent or alleviate it.
· Increased production. During a shortage, FDA may encourage existing manufacturers to increase production of the drug or encourage new manufacturers to seek approval to begin production of the drug in short supply. If more than one manufacturer produces the drug that is in short supply, FDA may contact the other manufacturers to alert them to the potential of increased demand and encourage them to increase their production of the drug.
· Expedite review of applications or inspections. FDA can expedite its review of an application or its inspections of a manufacturing establishment for manufacturers attempting to obtain FDA approval to restore, increase, or begin production of the shortage drug.[22] FDA can also expedite review of changes to an application to help prevent or mitigate a shortage, such as when the manufacturer wants to add a new production site or supplier, to ensure ongoing supply.
· Extend expiration dates. If a manufacturer provides data to support additional shelf life for a drug in shortage that is already in distribution and nearing its expiration date, FDA will review the data and, if found acceptable to support the additional shelf life, post information, including the extended date, on its drug shortage website.
· Work with manufacturer. FDA may work directly with the manufacturer to address the shortage depending on the specific problem and significance to patients. This effort may include additional mitigating controls implemented by the manufacturer as needed, or FDA providing feedback on a manufacturer’s proposal on how to address the shortage.
· Drug importation. When a manufacturer for the U.S. market is not able to resolve a shortage immediately and the shortage involves a critical drug needed for U.S. patients, FDA may look for a manufacturer in a foreign market willing and able to redirect that drug into the U.S. market to address the shortage. In this situation, FDA is to evaluate the drug to ensure efficacy and safety, including the quality of the manufacturing site where the drug is made.
· Compounding. Compounders prepare versions of an FDA-approved drug to meet individual patient needs. The compounded drug is not FDA-approved, and federal law generally restricts the marketing of drugs that are essentially copies of commercially available drugs.[23] However, when a drug is on FDA’s drug shortage list, some of these legal restrictions do not apply and a compounded copy of the drug in shortage can be marketed.
Other HHS agencies have a less direct role in addressing drug shortages but do play a role in the drug supply chain, and FDA may consult with these agencies, as appropriate, for input on topics outside of FDA’s purview. Examples include the following.
· ASPR has programs that fund domestic manufacturing for certain drug ingredients to strengthen the public health supply chain. For example, ASPR awarded approximately $11 million in 2024 to a company to domestically manufacture a drug ingredient that is critical to public health. Additionally, ASPR coordinated with FDA and other agencies to rapidly bolster domestic supply of intravenous solutions in response to Hurricanes Helene and Milton in 2024 by facilitating the importation of the drug from international sources, among other actions.
· The Centers for Medicare & Medicaid Services (CMS) implements certain payment policies and related requirements for prescription drugs through Medicare and Medicaid.[24] For example, through its role overseeing the Medicare program, CMS finalized a rule for fiscal year 2025 to encourage hospitals participating in the program to maintain a more reliable and resilient supply of essential medicines.[25] This rule offers increased Medicare payments to smaller, independent hospitals that maintain a 6-month stock of one or more essential medicines.[26]
Outside of HHS, DEA sets quotas for certain controlled substances—including certain prescription drugs—to help ensure their legitimate use while limiting potential abuse.
The federal government also purchases drugs for use in its own health care settings. The largest federal purchasers of drugs by expenditure are the Department of Defense (DOD) and the Department of Veterans Affairs (VA). Their purchasing is done generally through the same commercial market as other purchasers, such as hospitals and pharmacies.[27] In 2021, we described challenges DOD and VA faced in trying to examine the supply chains of their purchased drugs and meeting their goal to purchase drugs manufactured in the U.S.[28]
Drug Shortages Since the Start of the COVID-19 Pandemic Are Generally Consistent with Previous Trends
Since the start of the COVID-19 pandemic, the number and type of drugs in shortage generally continued pre-pandemic trends, but we found that the COVID-19 pandemic exacerbated previously existing supply chain vulnerabilities that underlie shortages. We also found that the categories of drugs in shortage prior to and after the COVID-19 pandemic have generally remained the same.
Annual Trends in New and Ongoing Drug Shortages Generally Continued Through the COVID-19 Pandemic
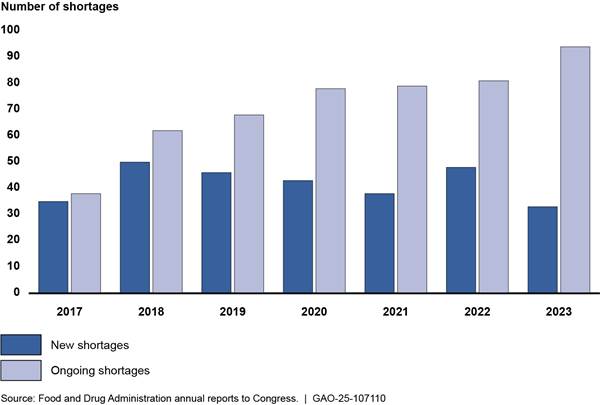
New and ongoing shortages. According to FDA, the number of new drug shortages per calendar year has generally decreased since 2020, when the COVID-19 public health emergency began (see fig. 2).[29] Conversely, the number of ongoing shortages yet to be resolved increased each year.[30]
Duration of shortages. We examined the duration of shortages using data
from the FDA Drug Shortage Database in two ways—when the shortages were ongoing
and when the shortages had been resolved.
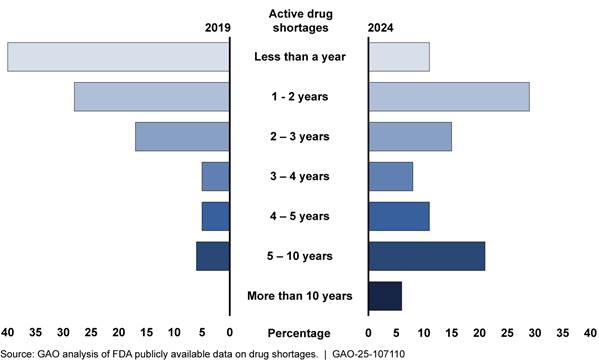
· For those shortages that were ongoing, the duration increased since 2019, indicating more persistent shortages, according to our analysis of FDA data (see fig. 3).[31] The increase in the duration of shortages has also been reported in other research. For example, one report found that the average duration of shortages increased from about 2 years in 2020 to 3 years in 2023.[32]
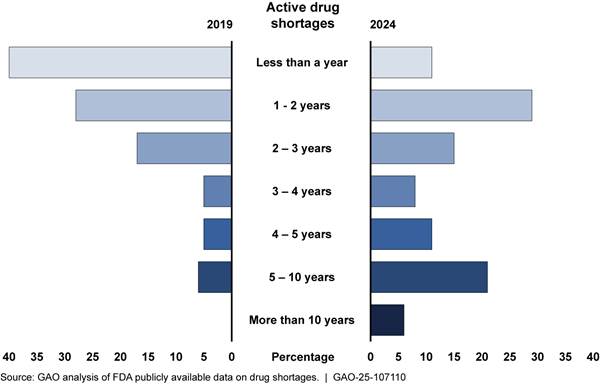
Note: As of December 29, 2019, there were 109 shortages on the Food and Drug Administration’s (FDA) drug shortage list. As of July 31, 2024, there were 102 shortages on FDA’s drug shortages list.
· FDA considers a shortage resolved when the drug supply is once again able to meet the national demand for the drug. Of the 103 shortages that were resolved between 2019 and 2023, the duration of resolved shortages did not vary greatly between these years. For example, 74 percent (29 of 39) of shortages resolved in 2019 were resolved in less than 2 years and 72 percent (21 of 29) of shortages resolved in 2021 were resolved in less than 2 years.
Persistent drug shortages may have negative effects on patient care. For example, a survey conducted by the American Cancer Society Cancer Action Network found that 22 percent of respondents in active cancer treatment were affected by drug shortages.[33] Further, the longer a shortage continues, the more patients are affected by either the need to receive substitute drugs or delays in treatment, according to two experts and patient advocacy groups we interviewed. One patient advocacy group representative we interviewed said patients would start experiencing these effects if a shortage lasted longer than 4 months.
Prevented shortages. The number of shortages that FDA has prevented each year has generally increased, including since the start of the COVID-19 pandemic, according to FDA annual reports. FDA considers a shortage to have been prevented when FDA is notified of a drug supply disruption and, due in part to actions FDA takes to address that disruption, the drug does not go into shortage. FDA data show that the number of shortages FDA prevented increased from 179 in 2020—when the COVID-19 pandemic started—to 224 in 2023. This continued a trend starting in 2014 when FDA reported 101 prevented shortages.
The COVID-19 Pandemic Exacerbated Supply Chain Issues and Other Reasons for Drug Shortages
FDA has identified ways that the COVID-19 pandemic exacerbated certain issues related to shortages.[34] For example, FDA stated that in 2021, 1 year after the start of the COVID-19 pandemic, there were no shortages directly connected to export restrictions enacted by other countries during the COVID-19 pandemic. However, the agency has stated that the pandemic has continued to affect the medical product supply chain, leading to shortages. For example, FDA indicated that the shortage of heparin was exacerbated by demand increases during the COVID-19 pandemic. Due to heparin’s anticoagulant properties, it can be used to reduce the risk of blood clotting caused by COVID-19, and its anti-inflammatory effects can reduce inflammation associated with the virus.
According to FDA, heparin pre-mixed bags of intravenous solutions went into shortage in 2017 due to a hurricane affecting drug manufacturing facilities in Puerto Rico. When the shortage was close to being resolved in 2020, a surge in demand associated with the COVID-19 pandemic extended it. In 2023, a tornado in North Carolina affected a warehouse that stored heparin pre-mixed bags of intravenous solutions, which further strained the supply chain. As of December 2024, heparin remained in shortage.
Nine of the 12 experts and industry representatives we interviewed said that the COVID-19 pandemic exposed weaknesses in the pharmaceutical supply chain and exacerbated existing shortages and supply chain issues. In addition, four of these experts and industry representatives said that the pandemic led to export restrictions and the shutdown of some manufacturing facilities, which may have indirectly contributed to shortages. For example, one of the 12 experts and industry representatives pointed towards a 2022 COVID-19 lockdown in China. This lockdown led to the shutdown of a manufacturing facility that produced an intravenous drug used during imaging to help doctors diagnose potential problems, such as organ infections and inflammation, by highlighting differences between soft tissues. The affected manufacturer produced more than half of the U.S. market share of this drug. This shutdown resulted in shortages and forced health care providers to implement conservation strategies.[35]
Prior to and after the COVID-19 pandemic, FDA has reported that a majority of shortages were caused by manufacturing quality issues. Specifically, FDA reported that 62 percent of shortages from 2013 through 2017 were due to manufacturing quality issues and has also indicated that quality continues to be a primary cause of shortages. FDA collects detailed information on the factors underlying each shortage, such as a manufacturing quality issue or extreme weather event interrupting supply, according to FDA officials. Quality issues underlie multiple statutory reasons for disruptions, according to FDA officials, as noted below. For example, “requirements related to complying with good manufacturing practices,” a reason reported on FDA’s list, is related to manufacturing quality. (See fig. 4 for the number and reasons for shortages as reported by FDA.)
Figure 4: Number of Drugs on FDA’s Shortage List by Reason for Shortage, as of December 2019, April 2021, and July 2024
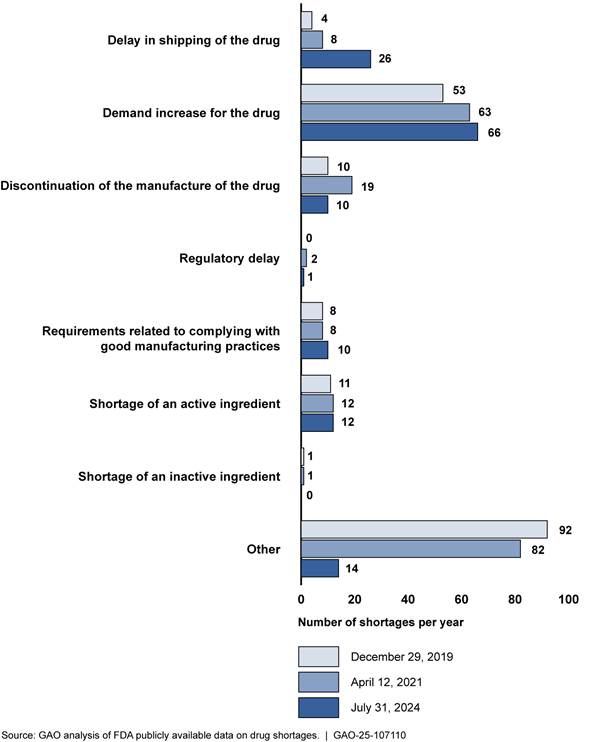
Notes: A single drug shortage could have multiple, different reasons reported, as drugs can have multiple suppliers that may experience different reasons why they are not able to meet demand. In this figure, we counted each unique reason for a shortage that manufacturers reported to the Food and Drug Administration (FDA) for each drug shortage. Therefore, there are more reasons represented in the figure than there are drugs in shortage during the time period depicted.
A single drug shortage may span multiple years. Therefore, the same shortage may be represented in more than one of the reported dates.
Federal law requires FDA to maintain a list of drugs that are in shortage; the agency must select a reason for the shortage from a list of categories defined in statute. 21 U.S.C. 356e(b)(3). When manufacturers submit a reason for the shortage to FDA, they must select a reason from that list. Manufacturers use the “other” category when the reason for a shortage cannot be categorized into one of the statutory reasons, according to FDA officials. For example, “other” may be used when the reason for a shortage is a quality issue, something that the statutory reasons do not adequately capture, according to FDA officials.
While quality is an underlying cause affecting the supply of drugs, according to our analysis of FDA data, increased demand was a reason for a number of drug shortages prior to the COVID-19 pandemic and continues to be a significant reason. More specifically, as of July 31, 2024, increased demand contributed to 65 percent (66 of 102) of shortages.[36] This continued an upward trend from 2019 and 2021, when increased demand was a reason for 49 percent (53 of 109) and 55 percent (63 of 115) of active shortages, respectively.[37] The only other statutory reason that considerably increased over this time period was delays in shipping of a drug, which increased from 4 percent (4 of 109) of shortages in 2019 to 7 percent (8 of 115) in 2021 and 25 percent (26 of 102) in 2024. FDA officials and six of the 12 experts and industry representatives we interviewed said that increased demand for drugs has been a factor in more shortages since the beginning of the COVID-19 pandemic. For example, the shortage of attention-deficit/hyperactivity disorder (ADHD) drugs that began in October 2022 was due to an increase in demand that a large ADHD drug manufacturer was not able to keep up with.
In addition to increased demand, “other” was frequently identified as a reason for shortages, though this decreased in 2024. The “other” category is used when the reason for a shortage cannot be categorized into one of the statutory reasons required by federal law, according to FDA officials. “Other” may be used, for example, when the reason for a shortage is a manufacturing delay or quality issue, something that the statutory reasons do not adequately capture, according to FDA officials.[38] “Other” reasons contributed to 84 percent (92 of 109) of shortages in 2019 and 14 percent (14 of 102) of shortages in 2024. FDA officials said they did not know why there had been a decrease in manufacturers’ use of “other” as a reason.
As previously mentioned, FDA can take a number of actions to help alleviate a drug shortage. For example, FDA may look for a foreign manufacturer that is willing and able to redirect product into the U.S. market to help address the shortage or reach out to other domestic manufacturers to see whether they can increase supply and fulfill demand. In the case of the ADHD drug shortage, FDA officials reached out to other manufacturers, but the overall supply could not be increased due to a variety of factors. Additionally, in 2024 when Hurricane Helene caused a shortage of intravenous solutions, FDA conducted scientific and regulatory assessments to help facilitate the temporary importation of intravenous solutions from some of the affected manufacturers’ facilities in other countries (see fig. 5).

While manufacturers are required to report to FDA an interruption in production caused by an increase in demand, they are not required to report when they see an increase in demand for a drug that does not cause an interruption in production.[39] Some manufacturers have voluntarily reported demand increases, according to FDA officials. However, these notifications are not routine, and FDA officials said that there have been instances of increased demand that were not reported to FDA by manufacturers and led to shortages. Earlier notification of when a manufacturer identifies a demand increase would help the agency act quickly, thus potentially preventing a shortage, according to FDA officials. For example, two manufacturers of an oncology drug that went into shortage in 2018 saw demand increases prior to that shortage, according to FDA officials. Because these manufacturers did not have an interruption in their manufacturing, they did not notify FDA of the demand increase. According to FDA officials, had these manufacturers notified FDA of the increase in demand for the drug prior to the shortage, FDA could have reached out to all manufacturers to address supply. The shortage lasted from January 2018 to June 2019.
Through its congressional budget justification for fiscal year 2025, FDA is seeking authority from Congress to require drug manufacturers to notify the agency when there is an increase in demand for a drug that the manufacturer will be unable to meet without meaningful shortfall or delay. FDA’s Chief Medical Officer also reiterated FDA’s request for this authority in a November 2024 hearing, stating that notice of increased demand would give FDA “more lead time in mitigating supply impacts.”[40]
Types of Drugs in Shortage as of 2024 Are Generally Consistent with Trends Prior to the COVID-19 Pandemic
According to our analysis of FDA data, the types of drugs in shortage prior to the start of the COVID-19 pandemic generally were still in shortage at the time of our work. In FDA’s drug shortage database, drugs are categorized by therapeutic category—the type of disease they are intended to treat. For example, cardiovascular drugs may be used to treat heart conditions, such as high blood pressure, while endocrinology drugs may be used to treat diseases affecting the endocrine system, including diabetes. A single drug may be assigned to more than one therapeutic category. In addition, across these categories, drugs delivered through injection—sterile injectables—were frequently in shortage. Four of twelve experts and industry representatives, and representatives from a patient advocacy group told us that shortages of these drugs can have a large effect on patient care.
Therapeutic categories of drugs. Similar therapeutic categories of drugs were in shortage both before and since the COVID-19 pandemic, although the magnitude of those shortages varied. For example, anesthesia and cardiovascular drugs were both in shortage before and after the pandemic, but the magnitude of the shortage changed (see fig. 6). After the pandemic, anesthesia drugs were the most common therapeutic category in shortage. Anesthesia drugs include drugs used to intubate patients with COVID-19.[41]
Figure 6: Number of Drugs on FDA’s Shortage List by Selected Therapeutic Categories, as of December 2019 and July 2024
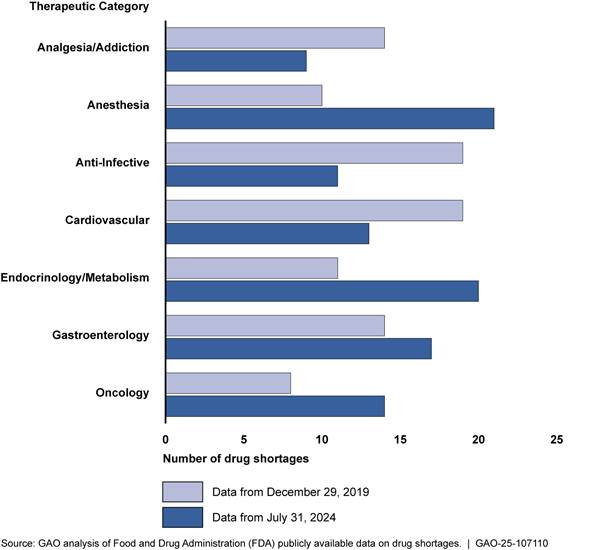
Note: A drug may be identified under more than one therapeutic category and more than one year.
Similarly, shortages of oncology drugs increased after the COVID-19 pandemic. Oncology drugs address many aspects of cancer treatment. According to our analysis of the 102 drug shortages as of July 31, 2024, 14 were oncology drugs. Five of the 12 experts and industry representatives we interviewed reported that oncology drugs are an important category of shortages. Two of the five experts and industry representatives noted that markets for these drugs are concentrated with only a few manufacturers producing the majority of the drugs in the market.[42]
Cancer patients have experienced negative effects due to oncology shortages, according to a survey conducted by the American Cancer Society Cancer Action Network.[43] The study found that 10 percent of respondents were patients in active treatment affected by a shortage, with over half related to the patient’s cancer treatments. Furthermore, for those patients facing a drug shortage related to their cancer treatment, just over two-thirds had difficulties finding substitute drugs and nearly half experienced delays or missed a treatment.
Cisplatin and carboplatin are two examples of oncology drugs that have been in shortage. Cisplatin and carboplatin are frequently used oncology drugs that have proven to be highly effective across a variety of cancer types (such as lung and breast cancer). Both drugs had five manufacturers when the shortages occurred, with a majority share of the cisplatin market concentrated with one manufacturer, according to FDA officials. Cisplatin went into shortage in 2023 after a cisplatin and carboplatin manufacturer closed their facility to address quality issues identified during an FDA inspection. This manufacturer held a large share of the cisplatin market, which caused a shortage. As the cisplatin shortage continued, health care providers switched to using carboplatin. The inability of the other four carboplatin manufacturers to keep up with this increased demand resulted in a shortage of carboplatin later in 2023. A survey of U.S. cancer centers in May 2023 found that 93 percent of the respondents were experiencing a shortage of carboplatin and 70 percent were experiencing a shortage of cisplatin. Further, 16 percent of the cancer centers said the carboplatin shortage resulted in patient treatment delays.[44]
To address the shortages, FDA officials stated they worked with the five cisplatin manufacturers to increase production, along with putting measures in place to ensure that the quality issues that had shut down manufacturing facilities were addressed. In addition, FDA worked with a sixth manufacturer to begin producing cisplatin again and evaluated a seventh manufacturer that was able to temporarily import cisplatin to meet patient needs during the shortage. FDA also worked with two additional carboplatin manufacturers to enter the market. The cisplatin shortage was resolved in June 2024, while carboplatin was still in shortage as of December 2024, according to FDA data. FDA continues to work with all seven manufacturers to resolve the carboplatin shortage, according to FDA officials.
Sterile injectable drugs. Of the 102 drugs we identified in active shortage as of July 31, 2024, 71 were sterile injectable drugs. Most drugs in shortage prior to the COVID-19 pandemic were also sterile injectables, according to our analysis of FDA data.
Sterile injectable drugs are found across a variety of therapeutic categories and are mainly used in hospital settings to treat a range of conditions. For example, some of the oncology drugs in shortage are sterile injectable drugs. In addition, shortages of injectable heparin, a hematology drug commonly kept in hospital crash carts to treat heart attacks, can greatly affect hospital staff’s ability to provide care to patients in life-threatening situations, according to a patient advocacy group we interviewed.[45] Sterile injectables—and generic versions of these, in particular—are commonly in shortage due to the complexity of manufacturing the drugs, and market factors, such as their low price and low-profit margins, according to eight of the 12 experts and industry representatives we interviewed. In 2014, we identified economic causes specific to the generic sterile injectable drug market, such as low profit margins leading to limited infrastructure investments or manufacturers exiting the market.[46] At that time, we found that 44 percent of critical shortages—drugs that do not have alternatives—involved generic sterile injectable drugs.[47]
Similarly, other research has emphasized the large number of sterile injectable drug shortages. For example, one study examining FDA drug shortage data from 2017 through June 2023, found that 67 percent of drugs in shortage were injectable drugs.[48]
FDA Is Taking Various Steps to Improve Its Capacity to Respond to and Help Prevent Shortages
FDA Is Taking Steps to Improve the Capacity of the Drug Shortage Staff
FDA is taking several steps to increase the Drug Shortage Staff’s capacity to respond to drug shortages by helping staff better manage workloads and decrease the burden of documenting their work responding to shortages.[49] The Drug Shortage Staff workload has increased since the COVID-19 pandemic. For example, according to an FDA annual report, the agency received 1,538 drug supply disruption notifications from manufacturers in 2023, compared to 473 notifications in 2020. FDA introduced a new portal for patients and providers to report drug shortages through FDA’s website. This portal led to a subsequent increase in workload caused by increased notifications, according to three of the five members of the Drug Shortage Staff we interviewed. Four of five Drug Shortage Staff members we interviewed said that, given their high workload, documenting their work to address a shortage is a secondary priority that can be burdensome.
FDA recently created two new project management positions within the Drug Shortage Staff to increase capacity by improving processes and reducing workload. FDA hired staff to fill these positions in April 2024. The first position was created to develop standard operating procedures to guide the work of the Drug Shortage Staff. These procedures will provide the Drug Shortage Staff with tools needed on a daily basis to do their work, such as guidance for interactions with stakeholders and a list of contacts, according to FDA officials. The second position was created to improve how activities are documented in Nexus, the workflow system used by Drug Shortage Staff, to ensure that future documentation is standardized and not burdensome for staff.[50] FDA officials stated they plan to have the new documentation processes finished and in place by early 2025.
Ensuring the Drug Shortage Staff have the capacity to respond to shortages and maintain documentation could help address a persistent data usability challenge for the agency. In 2011, we reported that FDA was unable to systematically monitor trends and identify actions it could take to address drug shortages because the agency lacked data on drug shortages, such as their causes and the agency’s response.[51] We recommended that FDA develop an information system that would allow drug shortage data to be tracked in a systematic manner. In response, the agency developed a database to track shortages, document the actions FDA takes in response, and monitor staff workload. However, in February 2014, we found these data were not standardized or accurate and further recommended that FDA develop policies and procedures to ensure staff enter information into the database in a consistent manner.[52] FDA subsequently developed these policies and procedures. However, in December 2022, FDA migrated from that system to the Nexus system in place at the time of our review.[53]
FDA Is Expanding Analytic Tools to Improve Its Response to Drug Shortages
FDA is expanding its data analytics tools to better analyze drug supply chains and potentially predict possible drug supply disruptions. This includes information systems and manufacturing data collection.
FDA is expanding information systems. FDA is developing new data analytics tools and is refining a tool it has used since 2021 to help the agency better analyze drug supply chain data to prevent and mitigate drug shortages.
In 2021, FDA introduced the Supply Chain Analytical Network System, a data system that helps staff to visualize, analyze, and aggregate product supply chains to assess potential drug shortages. For example, two of the five FDA Drug Shortage Staff members we interviewed said they used the system to obtain market share data on drugs that may go into shortage. The system compiles data, such as information on a manufacturer’s active pharmaceutical ingredient suppliers, that FDA extracts from various internal sources, including applications drug companies submit when they are seeking to market a drug and FDA inspection reports. FDA officials said the system will eventually provide a facility-level view of all drug products manufactured at a facility, but FDA currently lacks all the necessary data to do so. FDA officials said that they are working to incorporate additional information and sources, such as data on the total amount of each drug produced by each manufacturer.
In May 2024, FDA implemented the Insights Data Toolkit, a group of tools that expands upon the Supply Chain Analytical Network System to examine drug markets, not just drug supply chains. The Insights Data Toolkit compiles a variety of information, including FDA facility data, such as the location of manufacturing facilities, and historic shortage data, to visualize drug markets. According to FDA, the Insights Data Toolkit provides staff with high level metrics of drug products, including sales and market concentration data, and allows them to compare different market and supply chain data. (See fig. 7.)
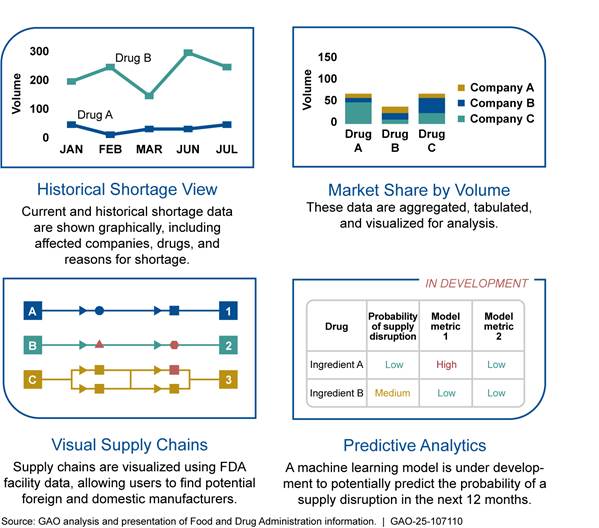
Note: These graphics are representations of possible visualizations in FDA’s Insights Data Toolkit and do not convey actual information.
As part of the development of the Insights Data Toolkit, FDA has also taken steps to develop the Supply Disruption Predictive Model to potentially identify supply disruptions that may lead to drug shortages. The model uses data from other FDA systems, including the Supply Chain Analytical Network System. FDA is validating the model, including assessing the accuracy of model’s predictions for the 2024 calendar year. According to FDA officials, the last round of validations will occur in May 2025. FDA has not yet set time frames for completion. FDA officials said that, once fully implemented, the model may provide the Drug Shortages Staff with an early signal of a potential supply disruption. The Drug Shortages Staff will then conduct their own analyses using the Insights Data Toolkit to find and address the cause of the early signal.
The development of the Supply Disruption Predictive Model is consistent with our 2014 recommendation that FDA conduct routine analyses of its drug shortage database to proactively identify risk factors for potential drug shortages early, thereby potentially helping FDA to recognize trends, clarify causes, and resolve problems before drugs go into short supply.[54] FDA agreed with this recommendation, and we will continue to monitor FDA’s implementation of the model for routine use.
FDA is taking steps to expand manufacturing data collection. FDA has started receiving more detailed manufacturing data intended to improve its understanding of manufacturing supply chains, but the agency’s implementation of this effort has faced challenges. The CARES Act directed manufacturers to annually report the total amount of drugs manufactured to FDA, starting in 2020.[55] Agency officials said these data may be able to provide insight into manufacturing supply chains and help determine whether a manufacturing disruption may lead to a drug shortage. These data will be incorporated into the Supply Chain Analytical Network System, according to FDA officials.
As of September 30, 2024, 44 percent of manufacturers of prescription drugs and 26 percent of over-the-counter drug manufacturers had submitted reports. FDA officials said they are unable to use the data collected until more manufacturers report and the agency is discussing ways to increase compliance. While FDA stated that manufacturers were required to submit reports beginning with calendar year 2020, FDA’s issuance of guidance was delayed. FDA did not implement the CARES Act requirement immediately, stating that the agency needed to determine the logistics for electronic reporting of the data and whether to incorporate reporting into existing data systems. FDA issued draft guidance to assist manufacturers in submitting reports to the agency on total amounts of drug manufactured in October 2021 and issued final guidance in February 2024.[56]
In addition, FDA and some of the experts and industry representatives we interviewed raised questions about the extent to which these data will be useful. As we reported in 2021, FDA officials initially expressed concern that this requirement would not likely provide complete insight into which suppliers are being used, because it does not expressly require manufacturers to identify sufficient details about the sources of ingredients used to manufacture drugs.[57] Six experts and industry representatives we spoke with also expressed concerns about how helpful the data would be to FDA. Specifically, reporting total annual amounts of drugs manufactured may not capture the entirety of the manufacturing process and therefore will not be helpful to FDA, according to three of these experts and industry representatives. For example, one expert stated that, because the same drug can be produced at multiple manufacturing facilities, a single manufacturing facility may not control how active pharmaceutical ingredients are sourced for all facilities manufacturing a certain drug. Therefore, a manufacturing facility may not have complete production information for a drug to report to FDA. Given these concerns, through its congressional budget justification for fiscal year 2025, FDA is seeking legislative authority to require enhanced reporting of supply chain data, such as information on manufacturers’ suppliers and the extent to which the suppliers are relied on. These data are intended to address some of the gaps that the agency identified with the data that it is currently receiving.
Utilizing these data and seeking additional authority would be consistent with our 2021 recommendation that FDA make changes to its collection of drug manufacturing data to ensure the information obtained is complete and accessible to help it identify and mitigate supply chain vulnerabilities.[58] FDA neither agreed nor disagreed with this recommendation. We are monitoring FDA’s collection and use of the data that it is currently collecting as we assess FDA’s progress to implement this recommendation.
FDA Is Developing Efforts That Are Intended to Help Improve Manufacturer Resilience to Prevent Drug Shortages
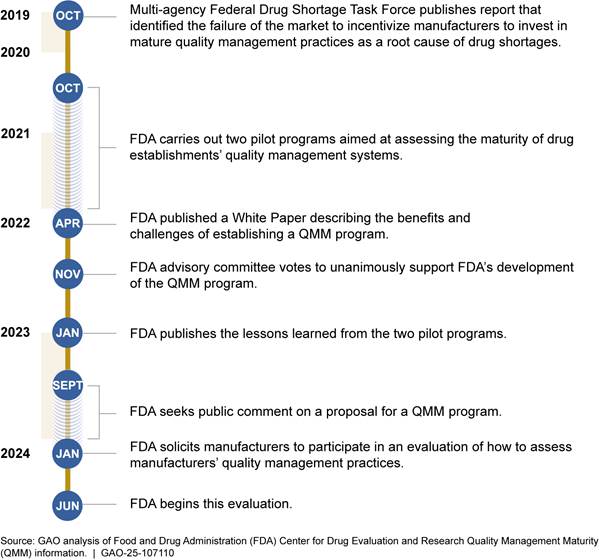
FDA is developing two efforts intended to help prevent supply disruptions through improved quality management practices—the Quality Management Maturity (QMM) program and manufacturer risk management plans.[59] Both efforts are in the early stages, and it is not yet clear what effect they will have on drug shortages if fully implemented as intended.
QMM program. FDA began developing the QMM program in 2020 to encourage drug manufacturers to implement quality management practices to support a more reliable drug supply chain. A QMM program, according to FDA, attempts to address one of the root causes of drug shortages identified in an FDA-led, inter-agency Drug Shortages Task Force root causes report—a market that does not reward manufacturers for having more mature quality management systems.[60] Quality-related issues have been, and continue to be, one of the leading reported causes of drug shortages. QMM could be used to help incentivize drug manufacturers to invest in quality management maturity practices. According to FDA, QMM practices would go beyond the minimum quality requirements that FDA already has for manufacturers.[61] FDA indicated that promoting more mature quality management practices will support a more reliable drug supply chain by reducing the occurrence of quality-related failures at manufacturing sites and improve the ability of manufacturers to maintain production during expected and unexpected supply chain disruptions.[62]
According to agency officials, FDA does not have statutory authority to offer financial incentives to encourage manufacturers to invest in quality management maturity, but it may be possible that QMM program outcomes could be used in conjunction with other programs to encourage investment in more mature quality management practices. For example, in April 2024, HHS indicated that it is working on facilitating greater transparency so the market can use information about mature quality management practices to reward resilience.[63] FDA officials said their goal is to develop a reliable measure of a manufacturer’s quality management maturity practices, but defer to other HHS agencies on how that measure would be used to influence purchasing or other factors outside FDA’s control. (See text box for a quality management program for medical devices that FDA created in 2011.)
|
Food and Drug Administration (FDA) Device Quality Management Program
FDA created a quality management
program for medical devices in 2011. The program was intended to encourage
medical device manufacturers to shift their focus beyond baseline regulatory
compliance to sustained, predictive practices that advance medical device
quality and safety. In deciding to develop the program, FDA found there was a
lack of data around quality that would enable market drivers to reward
high-quality manufacturing and continuous improvement. Once FDA identified an
evaluation system, it launched a voluntary pilot program to assess device
manufacturing quality maturity in 2018. Results of the pilot showed, for example, increases to safety through the implementation of manufacturing improvements and improved access through increased production capacity. Pilot participants also reported hundreds of thousands of dollars in operational improvements and millions of dollars in revenue opportunities. FDA issued final guidance for this voluntary program in September 2023. FDA officials involved with the drug Quality Management Maturity (QMM) program said that they are aware of the program for medical devices and will pursue appropriate options for use as a model for the QMM program. Source: GAO analysis of FDA information. | GAO‑25‑107110 |
The QMM program is still under development, and FDA officials said they have not made final decisions about its design and use, including how to assess the quality management maturity practices for manufacturers that participate. FDA officials said that participation from manufacturers is likely to be voluntary, and FDA will rely on manufacturers to seek this additional assessment. FDA is working on how to incentivize manufacturers to participate in the program. According to FDA officials, once FDA develops the program, the intention is that manufacturers would be able to decide if they would like to make their participation and assessment public. FDA officials said manufacturers may be more willing to both participate in the QMM program and make their assessments public if there are incentives to doing so.
Experts and industry representatives we interviewed said FDA’s decisions about the QMM program’s design and use could have ramifications for whether it is effective in preventing drug shortages. For example, according to five of the 12 experts and industry representatives we interviewed, it is still not clear how ratings from the program will be used, as it is unknown whether there would be incentives for drug purchasers to use these ratings when making decisions on which drugs to purchase. Four of the 12 experts and industry representatives we spoke with expressed doubt about drug purchasers’ willingness to pay more for quality manufacturing.
FDA conducted two pilots for the QMM program between 2020 and 2022 that allowed FDA to test how it would assess manufacturers’ quality management practices. In June 2024, FDA began an evaluation of its assessment practices at nine volunteer manufacturers, according to agency officials. FDA officials said the agency plans to complete these assessments by the end of 2024 and then analyze the results in 2025. FDA officials said they do not yet have clear time frames for full implementation of the QMM program. Implementation of the program is contingent on several factors, including refinement of the assessment process and obtaining adequate resources to support the program, according to FDA officials. See figure 8 for a timeline of the QMM program.
Risk management plans. FDA issued draft guidance in May 2022 on how
manufacturers can implement the CARES Act requirement to develop, maintain, and
implement risk management plans.[64]
These plans can provide manufacturers with a framework to identify, prioritize,
and implement strategies proactively to mitigate hazards that can lead to drug
shortages. Hazards include extreme weather events, cyberattacks, and other
events that could lead to manufacturing and supply disruptions. For example, in
2017, Hurricane Maria disrupted manufacturing in Puerto Rico, leading to
widespread shortages of intravenous saline, a product used for the delivery of
care in hospitals.
According to the FDA draft guidance, risk management plans should contain a broader strategy that establishes overarching approaches to consistently identify, assess, and mitigate risk, which is consistent with established quality management practices. These plans could preemptively reduce the financial and resource burden associated with resolving a shortage and reduce problems that may lead to a shortage, according to FDA. As of August 2024, FDA officials said they do not have time frames for issuing final guidance as the agency is still reviewing comments on the draft guidance, but they plan to issue the final guidance as quickly as possible. FDA has not indicated whether it will assess the risk management plans, though they said the risk management plans may be reviewed as part of an inspection.
Four experts and industry representatives we interviewed generally said drug manufacturers were already likely to have risk management plans in place, and that these were in effect during the COVID-19 pandemic and recent extreme weather events. One of the experts and industry representatives said that, to the extent that some manufacturers did not have such plans in place prior to the CARES Act, the requirement could help prevent and mitigate shortages. However, a different representative we interviewed noted that some generic drug manufacturers may be aware of and accepting these risks. They said this is true given the low profitability of the drugs they manufacture. Another expert and industry representative noted the additional costs associated with having risk management processes in place, such as redundancy in their supply chains.
The Current Drug Shortage Coordinator Position Will End in Mid-2025, Leaving No Coordination Mechanism
As part of a broader effort to mitigate drug shortages, President Biden announced the creation of an HHS Supply Chain Resilience and Shortages Coordinator in November 2023.[65] HHS designated the Coordinator to be within the Office of the Assistant Secretary for Planning and Evaluation (ASPE).[66] The Coordinator position was initially funded for 4 years, according to ASPE officials. The Coordinator was to coordinate certain drug shortage and supply chain resilience activities across HHS, according to ASPE officials. ASPE appointed an acting Coordinator who began taking steps to coordinate drug shortages activities across the department. For example, ASPE took steps to hire a Coordinator, created an HHS Supply Chain Workgroup, developed a draft action plan, and outlined a proposed budget.[67]
HHS officials told us in February 2025 that funding for the Coordinator position and related functions would expire in May 2025. Therefore, the activities of the Coordinator and the Workgroup, including the draft action plan, would end at that point.
When the Coordinator and its functions end in May 2025, HHS will no longer have a mechanism to coordinate drug shortage activities across the department. This will return HHS to its prior state before the Coordinator, when HHS had no formal coordinating structure to oversee department-wide responses and strategies. This limited the capability of HHS to mitigate and respond to shortages and strengthen supply chain resilience, according to HHS. FDA, Congress, and academic experts have reported that collaboration across the federal government is important to address drug shortages and enhance supply chain resiliency, because each agency has a unique role in the supply chain.[68]
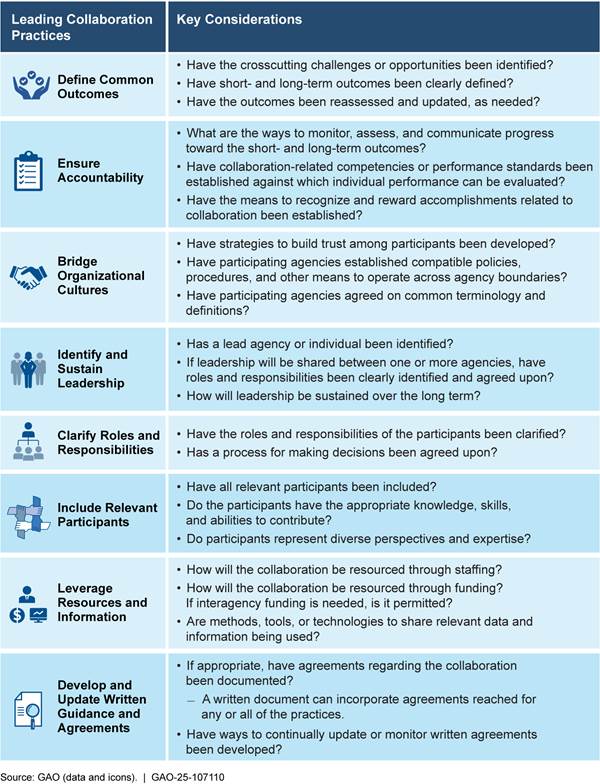
To mitigate and respond to drug shortages, it will be important for HHS to identify and implement a mechanism to coordinate its strategies across the department, and to collaborate with other federal stakeholders. Our eight leading practices for interagency collaboration provide key considerations that help agencies achieve important interagency outcomes (see app. I for more information on these leading practices).[69] Leading practices such as identifying and sustaining leadership and including relevant stakeholders, among others, will be important for HHS to consider when implementing a mechanism to coordinate the department’s drug shortage activities.
|
Key Considerations for Identifying and Has a lead agency or individual been identified? If leadership will be shared between one or more agencies, have roles and responsibilities been clearly identified and agreed upon? How will leadership be sustained over the long term?
Source: GAO (data and icons). | GAO‑25‑107110 |
Identifying and sustaining leadership. According to our leading practices for interagency collaboration, strong and sustained leadership helps interagency efforts to function. Without a leadership figure to provide authority and facilitate decision making, collaborative efforts may be weakened or ineffective. ASPE had designated the Coordinator as a key leadership figure, and, as the chair of the HHS Supply Chain Workgroup, the Coordinator interacted with several agencies within HHS, according to ASPE officials. Absence of the Coordinator position after May 2025 will leave HHS without a formal coordinating structure or a leader to oversee drug shortage activities across the department.
|
Key Considerations for Relevant Participants Have all relevant participants been included? Do the participants have the appropriate knowledge, skills, and abilities to contribute? Do the participants represent diverse perspectives and expertise?
Source: GAO (data and icons). | GAO‑25‑107110 |
Including relevant stakeholders. According to our leading practices for interagency collaboration, the inclusion of relevant participants requires agencies to invite all relevant organizations who may have a stake in the collaborative effort. ASPE included representatives from multiple HHS agencies, such as ASPR and CMS, in the HHS Supply Chain Workgroup. Outside of the Workgroup, these agencies have varying roles that may address drug shortages and enhance supply chain resilience, but each have certain limitations. For example, FDA is limited in its ability to provide manufacturers with financial incentives or require manufacturers to increase production; however, ASPR has provided funding to manufacturers to increase domestic production of certain drug substances and drug products.
It will also be important to consider the role of departments outside of HHS. While the Coordinator was limited to drug shortage activities within HHS, other departments outside of HHS have a role in shortages and the supply chain. Examples of other relevant stakeholders outside of HHS include the following.
· DEA. DEA sets limits on the amount of a controlled substance, such as ADHD drugs, that can be manufactured. FDA coordinates with DEA to address shortages of drugs containing these controlled substances.
· Federal Emergency Management Agency. The Federal Emergency Management Agency within the Department of Homeland Security leads and coordinates federal response efforts in cases of emergencies, such as an extreme weather event or a pandemic. ASPE used data from the Federal Emergency Management Agency to determine the risk of extreme weather affecting a manufacturing location, which could affect the supply chain.
· DOD and VA. DOD and VA are large federal purchasers of drugs with each agency responsible for the delivery of drugs to over 9 million beneficiaries. In addition, HHS previously collaborated with DOD and VA on the National Strategy for a Resilient Public Health Supply Chain to design, build, and sustain long-term capability in the U.S. to manufacture supplies for future pandemics and biological threats.[70]
Other federal efforts to address drug shortages have involved coordinating with these and other relevant stakeholders. For example, FDA’s Drug Shortages Task Force included representatives from HHS, DOD, and VA. With many relevant stakeholders inside and outside of HHS, it will be important for HHS to identify and include these stakeholders in its future efforts to coordinate drug shortage efforts.
In written comments, the agency stated that the current administration had not indicated how it will direct and coordinate supply chain activities moving forward. This includes coordinating drug shortages once the Coordinator and its functions end in May 2025. HHS and others have stated that coordination of drug shortage activities is important to address this serious public health issue. Our leading practices for interagency collaboration state that effective collaboration between agencies and coordination is critical to achieving important interagency outcomes. Therefore, it will be important for HHS to identify and implement a mechanism that it will use to formally coordinate its drug shortage activities.
Conclusions
FDA plays an important role in responding to drug shortages and has made progress in preventing these shortages. Although FDA may be able to address some immediate causes of drug shortages, the agency cannot control many of the underlying economic factors that create a lack of incentives to produce less profitable drugs and invest in quality or the logistical and regulatory challenges that make it difficult for manufacturers to increase production. These factors contribute to manufacturing quality issues and may reduce a manufacturer’s motivation to improve their product quality or stay in the market to ensure redundancy in the supply chain.
This issue is complex and FDA, Congress, and others have reported on the importance of collaboration across the federal government to address drug shortages and enhance supply chain resiliency. To ensure coordination, it will be important for HHS to identify and implement a new mechanism to do so. This mechanism should take GAO’s leading practices for interagency collaboration into consideration to ensure effective collaboration with the multiple agencies that have responsibilities pertaining to drug shortages, both inside and outside of HHS. Ensuring HHS engages in this collaboration to address drug shortages and supply chain resiliency will be an important step to addressing this critical public health issue.
Recommendation for Executive Action
The Secretary of Health and Human Services should identify and implement a mechanism to formally coordinate HHS strategies to address drug shortages and collaborate with other federal stakeholders, as needed. (Recommendation 1)
The Secretary of Health and Human Services should ensure this mechanism takes into consideration GAO’s Leading Practices to Enhance Interagency Collaboration and Address Crosscutting Challenges as it carries out its activities to address drug shortages. (Recommendation 2)
Agency Comments and Our Evaluation
We provided a draft of this report to HHS for review and comment. HHS provided technical comments, which we incorporated as appropriate. We also received written comments from HHS, which are reprinted in appendix II.
We made significant changes to this report and the corresponding recommendation as a result of the new information HHS provided in its comments, specifically about the termination of the Coordinator position and its functions. In the draft report that HHS reviewed, we recommended that ASPE document how it would implement our Leading Practices to Enhance Interagency Collaboration and Address Crosscutting Challenges as the Supply Chain Resilience and Shortage Coordinator and HHS Supply Chain Workgroup completed the action plan. In its written comments, HHS stated that it did not concur with our recommendation, as the Coordinator position and its associated functions would be ending in May 2025, and the current administration had not indicated how it will direct and coordinate supply chain activities moving forward.
Drug shortages are a serious public health issue that can affect some of the most vulnerable patients. Given the role of multiple agencies in addressing drug shortages and securing the supply chain, coordination remains important, as FDA cannot address these issues on its own. Therefore, we are recommending that HHS identify and implement a mechanism to formally coordinate drug shortage strategies and ensure this mechanism takes into consideration our leading practices.
We are sending copies of this report to the appropriate congressional committees, the Secretary of Health and Human Services, and other interested parties. In addition, the report is available at no charge on the GAO website at https://www.gao.gov.
If you or your staff have any questions about this report, please contact me at DeniganMacauleyM@gao.gov. Contact points for our Offices of Congressional Relations and Public Affairs may be found on the last page of this report. GAO staff who made key contributions to this report are listed in appendix III.

Mary Denigan-Macauley
Director, Health Care
List of Addressees
The Honorable Susan Collins
Chair
The Honorable Patty Murray
Vice Chair
Committee on Appropriations
United States Senate
The Honorable Mike Crapo
Chairman
The Honorable Ron Wyden
Ranking Member
Committee on Finance
United States Senate
The Honorable Bill Cassidy, M.D.
Chair
The Honorable Bernie Sanders
Ranking Member
Committee on Health, Education, Labor, and Pensions
United States Senate
The Honorable Rand Paul, M.D.
Chairman
The Honorable Gary C. Peters
Ranking Member
Committee on Homeland Security and Governmental Affairs
United States Senate
The Honorable Tom Cole
Chairman
The Honorable Rosa L. DeLauro
Ranking Member
Committee on Appropriations
House of Representatives
The Honorable Brett Guthrie
Chairman
The Honorable Frank Pallone, Jr.
Ranking Member
Committee on Energy and Commerce
House of Representatives
The Honorable Mark E. Green, M.D.
Chairman
The Honorable Bennie G. Thompson
Ranking Member
Committee on Homeland Security
House of Representatives
The Honorable James Comer
Chairman
The Honorable Gerald E. Connolly
Ranking Member
Committee on Oversight and Government Reform
House of Representatives
The Honorable Jason Smith
Chairman
The Honorable Richard Neal
Ranking Member
Committee on Ways and Means
House of Representatives
The Honorable Timothy M. Kennedy
House of Representatives
Note: For more information on GAO’s leading practices, see GAO, Government Performance Management: Leading Practices to Enhance Interagency Collaboration and Address Crosscutting Challenges, GAO‑23‑105520 (Washington, D.C.: May 24, 2023).


GAO Contact
Mary Denigan-Macauley, Director, Health Care, DeniganMacauleyM@gao.gov
Staff Acknowledgment
In addition to the contact named above, William Hadley (Assistant Director), Sarah Resavy (Analyst-in-Charge), Sylvia Diaz Jones, Jack Knauer, and Ashley Nurhussein made key contributions to this report. Also contributing were Sonia Chakrabarty, Sang Lee, Laurie Pachter, Ethiene Salgado-Rodriguez, and Ravi Sharma.
The Government Accountability Office, the audit, evaluation, and investigative arm of Congress, exists to support Congress in meeting its constitutional responsibilities and to help improve the performance and accountability of the federal government for the American people. GAO examines the use of public funds; evaluates federal programs and policies; and provides analyses, recommendations, and other assistance to help Congress make informed oversight, policy, and funding decisions. GAO’s commitment to good government is reflected in its core values of accountability, integrity, and reliability.
Obtaining Copies of GAO Reports and Testimony
The fastest and easiest way to obtain copies of GAO documents at no cost is through our website. Each weekday afternoon, GAO posts on its website newly released reports, testimony, and correspondence. You can also subscribe to GAO’s email updates to receive notification of newly posted products.
Order by Phone
The price of each GAO publication reflects GAO’s actual cost of production and distribution and depends on the number of pages in the publication and whether the publication is printed in color or black and white. Pricing and ordering information is posted on GAO’s website, https://www.gao.gov/ordering.htm.
Place orders by calling (202) 512-6000, toll free (866) 801-7077,
or
TDD (202) 512-2537.
Orders may be paid for using American Express, Discover Card, MasterCard, Visa, check, or money order. Call for additional information.
Connect with GAO
Connect with GAO on X,
LinkedIn, Instagram, and YouTube.
Subscribe to our Email Updates. Listen to our Podcasts.
Visit GAO on the web at https://www.gao.gov.
To Report Fraud, Waste, and Abuse in Federal Programs
Contact FraudNet:
Website: https://www.gao.gov/about/what-gao-does/fraudnet
Automated answering system: (800) 424-5454
Media Relations
Sarah Kaczmarek, Managing Director, Media@gao.gov
Congressional Relations
A. Nicole Clowers, Managing Director, CongRel@gao.gov
General Inquiries
[1]See 21 U.S.C. § 356c(a)-(b). Manufacturers of drugs that are life-supporting, life sustaining, or used to prevent or treat debilitating health issues are required to notify FDA of a permanent discontinuance of a drug or an interruption in its manufacture that is likely to lead to a meaningful disruption in the drug’s supply in the U.S. Manufacturers must notify FDA at least 6 months prior to the date of a discontinuance or interruption (or as soon as practicable if 6 months’ notice is not possible).
[2]We previously reported on FDA and drug shortages, including trends in the number, causes, and duration of shortages; FDA’s prioritization of reviews of drug submissions to address drug shortages; and the progress FDA has made on addressing drug shortages. See GAO, Drug Shortages: Certain Factors Are Strongly Associated with This Persistent Public Health Challenge, GAO‑16‑595 (Washington, D.C.: July 7, 2016); Drug Shortages: Better Management of the Quota Process for Controlled Substances Needed; Coordination between DEA and FDA Should Be Improved, GAO‑15‑202 (Washington, D.C.: Feb. 2, 2015); Drug Shortages: Public Health Threat Continues, Despite Efforts to Help Ensure Product Availability, GAO‑14‑194 (Washington, D.C.: Feb. 10, 2014); and Drug Shortages: FDA’s Ability to Respond Should Be Strengthened, GAO‑12‑116 (Washington, D.C.: Nov. 21, 2011).
[3]See GAO‑14‑194.
[4]GAO, High-Risk Series: Heightened Attention Could Save Billions More and Improve Government Efficiency and Effectiveness, GAO‑25‑107743 (Washington, D.C.: Feb. 25, 2025).
[5]In November 2023, President Biden also broadened HHS’s authorities under the Defense Production Act to enable investment in domestic manufacturing of essential medicines and medical countermeasures. See White House, Fact Sheet: President Biden Announces New Actions to Strengthen America’s Supply Chains, Lower Costs for Families, and Secure Key Sectors (Washington, D.C.: Nov. 27, 2023), https://bidenwhitehouse.archives.gov/briefing-room/statements-releases/2023/11/27/fact-sheet-president-biden-announces-new-actions-to-strengthen-americas-supply-chains-lower-costs-for-families-and-secure-key-sectors/.
[6]See the New High-Risk Designation: HHS and Public Health Emergencies appendix in GAO, COVID-19: Significant Improvements Are Needed for Overseeing Relief Funds and Leading Responses to Public Health Emergencies, GAO‑22‑105291 (Washington, D.C.: Jan. 27, 2022).
[7]Specifically, the act requires us to monitor and oversee the federal government’s efforts to prepare for, respond to, and recover from the pandemic. Pub. L. No. 116-136, § 19010(b), 134 Stat. 281, 580 (2020). The American Rescue Plan Act of 2021 also includes a provision for us to conduct oversight of the COVID-19 response. Pub. L. No. 117-2, § 4002, 135 Stat. 4, 78. All of GAO’s reports related to the COVID-19 pandemic are available on GAO’s website at https://www.gao.gov/coronavirus.
[8]The focus of this report is on shortages of drugs for human use that are regulated by FDA’s Center for Drug Evaluation and Research.
[9]See GAO‑16‑595.
[10]White House, Fact Sheet: President Biden Announces New Actions to Strengthen America’s Supply Chains, Lower Costs for Families, and Secure Key Sectors (Washington, D.C.: Nov. 27, 2023).
[11]See GAO, Government Performance Management: Leading Practices to Enhance Interagency Collaboration and Address Crosscutting Challenges, GAO-23-105520 (Washington, D.C.: May 24, 2023).
[12]These organizations were the American Society of Health-System Pharmacists, University of Utah Drug Information Service, Brookings Institution, US Pharmacopeia, Association for Accessible Medicines, Pharmaceutical Research and Manufacturers of America, Manhattan Institute, Duke-Margolis Center for Health Policy, Pharma and Biopharma Outsourcing Association, Bulk Pharmaceuticals Task Force, American Hospital Association, and American Medical Association.
[13]The groups we interviewed were Angels for Change, AARP, and the American Cancer Society Cancer Action Network.
[14]See 21 U.S.C. § 356c(h)(2).
[15]The Drug Shortages Task Force included officials from FDA, Centers for Medicare & Medicaid Services (CMS), Department of Defense (DOD), Department of Veterans Affairs (VA), Federal Trade Commission, and ASPR. See Food and Drug Administration, Drug Shortages: Root Causes and Potential Solutions (2019).
[16]If the opportunity cost of entering or staying in the market for a drug is higher than current or expected profits, manufacturers may switch to producing more profitable drugs in the medium- and longer-term or cease production to minimize losses. This may be the case for some low-cost generic drugs, which compete primarily based on price.
[17]See 21 U.S.C. § 356e.
[18]See 21 U.S.C. § 356c(a).
[19]The Drug Shortage Staff was established in 1999 in FDA’s Center for Drug Evaluation and Research, which regulates over-the-counter and prescription drugs, including biological therapeutics and generic drugs.
[20]A drug is medically necessary if it is used to treat or prevent a serious disease or medical condition for which an acceptable alternative drug product or therapy is not available. In general, the Drug Shortage Staff focuses on shortages of medically necessary drugs that have a significant effect on public health.
[21]See 21 U.S.C. § 356c(a). Federal law requires FDA to maintain a list of drugs that are in shortage; the agency must select a reason for the shortage from a list of categories defined in statute. 21 U.S.C. § 356e(b)(3). Manufacturers must select one of the statutory reasons or “other” when providing information for a drug on the drug shortage list.
[22]To obtain FDA’s approval of a new drug application, sponsors must submit, among other things, data on the safety and effectiveness of the new drug. Sponsors submitting an application for a generic drug must submit data that satisfies the regulatory requirements to establish bioequivalence and meet other approval requirements under relevant statutes. As part of the application review process, FDA may conduct an inspection of the establishment where the drug will be manufactured to verify the accuracy and authenticity of the data contained in the application, determine that the establishment is following commitments made in the application, and verify that the establishment is prepared to make the drug named in the application.
[23]Drug compounding is the process by which a pharmacist or doctor combines, mixes, or alters ingredients to create a drug tailored to the medical needs of an individual patient. Drugs compounded by a licensed pharmacist in a state-licensed pharmacy or federal facility, or by a licensed physician, and that meet certain statutory requirements, can qualify for exemptions from the FDA approval requirements, the requirement to label products with adequate directions for use, and the requirement to comply with current good manufacturing practice requirements. See 21 U.S.C. § 353a(a). Federal law restricts compounding drugs that are essentially copies of commercially available drugs, but a drug is not considered to be commercially available if it is on FDA’s shortage list. See 21 U.S.C. § 353a(b). For more information on drug compounding see GAO, Drug Compounding: FDA Has Taken Steps to Implement Compounding Law, but Some States and Stakeholders Reported Challenges, GAO‑17‑64 (Washington, D.C.: Nov. 17, 2016).
[24]Medicare is the federally financed health insurance program for persons aged 65 and over, certain individuals with disabilities, and individuals with end-stage renal disease. Medicaid is a joint federal-state program that finances health care for certain low-income and medically needy individuals.
[25]Executive Order 13944 directed FDA to identify a list of essential medicines, which are those that are medically necessary to have available at all times in an amount adequate to serve patient needs and in the appropriate dosage forms. Executive Office of the President, Combating Public Health Emergencies and Strengthening National Security by Ensuring Essential Medicines, Medical Countermeasures, and Critical Inputs Are Made in the United States, § 3(c), 85 Fed. Reg. 49,929 (Aug. 6, 2020). FDA created a list of 227 drug and biologic products that were essential in accordance with section 3(c) of the order. See Food and Drug Administration, Drug and Biologic Essential Medicines, Medical Countermeasures, and Critical Inputs (Oct. 30, 2020).
The CMS rule used 86 critical drugs selected through an ASPR-led National Forum to Secure America’s Supply Chain for Essential Medicines in response to Executive Order 14017. Executive Office of the President, America’s Supply Chains, 86 Fed. Reg. 11,849 (Feb. 24, 2021). This list of critical drugs was drawn from FDA’s essential medicines list with a focus on enhancing domestic manufacturing efforts and to help develop strategies to overcome current drug supply chain challenges and constraints. See National Forum to Secure America’s Supply Chain for Essential Medicines, Essential Medicines Supply Chain and Manufacturing Resilience Assessment (Manchester, N.H.: Advanced Regenerative Manufacturing Institute, 2022), https://www.armiusa.org/biofabusa/roadmap-reports.
[26]See Centers for Medicare & Medicaid Services, Hospital Inpatient Prospective Payment System and Long-Term Care Hospital Prospective Payment System, 89 Fed. Reg. 68,986 (Aug. 28, 2024).
[27]The U.S. does not have an overarching national pricing strategy for prescription drugs, although some of its publicly funded coverage, such as Medicaid and VA, use their own pricing strategies, such as pricing formulas. GAO, Prescription Drugs: U.S. Prices for Selected Brand Drugs Were Higher on Average than Prices in Australia, Canada, and France, GAO‑21‑282 (Washington, D.C.: Mar. 29, 2021).
[28]GAO, COVID-19: Critical Vaccine Distribution, Supply Chain, Program Integrity, and Other Challenges Require Focused Federal Attention, GAO‑21‑265 (Washington, D.C.: Jan. 28, 2021).
[29]A new shortage is a shortage that began during the reporting year, according to FDA officials.
[30]An ongoing shortage is a shortage that began in a prior year and was still active as of December 31 of the reporting year, according to FDA officials. Therefore, FDA’s count of ongoing shortages does not include shortages that began in a prior year but were resolved before December 31 of the reporting year.
[31]We previously reported that, between January 1, 2007, and June 30, 2013, 68 percent of shortages lasted 1 year or less. See GAO‑14‑194.
[32]United States Pharmacopeia, USP Annual Drug Shortages Report: Economic Factors Underpin 2023 Shortages (2024).
[33]American Cancer Society Cancer Action Network, Survivor Views: Drug Shortages, Telehealth, & Biomarker Testing (2023).
[34]While FDA has identified ways the COVID-19 pandemic exacerbated certain issues related to drug shortage, the agency has not systematically tracked whether specific shortages were caused by the pandemic.
[35]David R. Koeppel and Ingrid B. Boehm, “Shortage of Iodinated Contrast Media: Status and Possible Chances - A Systematic Review,” European Journal of Radiology, vol.164 (2023). Intravenous contrast media are drugs containing iodine that are given to patients to enhance the ability to see blood vessels and organs on medical images such as X-rays or computed tomography scans. These images provide greater detail when necessary to help health care professionals diagnose potential problems.
[36]There can be multiple reasons for a single shortage. According to our analysis of FDA data as of July 31, 2024, there was more than one reported reason for 73 percent of active shortages. Similarly, our analysis of FDA data from before the COVID-19 pandemic (December 29, 2019) and during the COVID-19 pandemic (April 12, 2021) showed that manufacturers reported more than one reason for 70 percent of active shortages for each time period. While we cannot determine how each reported reason affects a shortage, for the purpose of our analysis we assumed that citing a reason as the only reason is equivalent to citing that reason along with additional reasons.
[37]We previously reported that 6 percent of the shortages reported between January 1, 2011, and June 30, 2013, were due to increased demand. See GAO‑14‑194.
[38]We previously reported that 40 percent of the shortages reported between January 1, 2011, and June 30, 2013, were due to a quality problem and 30 percent were due to manufacturing delays and capacity issues. See GAO‑14‑194.
[39]FDA can select demand increase for the drug as one of the reasons for a shortage listed in statute. 21 U.S.C. § 356e(b)(3).
[40]Dr. Hilary Marston, Food and Drug Administration Chief Medical Officer, Preparing for the Next Pandemic: Lessons Learned and the Path Forward, testimony before the Select Subcommittee on the Coronavirus Pandemic, 118th Cong., November 14, 2024.
[41]In FDA’s drug shortage database, drugs are categorized by the type of disease they are intended to treat. For example, cardiovascular drugs may be used to treat heart conditions, such as high blood pressure. A single drug may be assigned to more than one therapeutic category.
[42]We previously reported on the concentrated market for oncology drugs. Specifically, that in 2008, three manufacturers produced 71 percent of all generic sterile injectable oncology drugs. See GAO‑14‑194.
[43]American Cancer Society Cancer Action Network, Survivor Views: Drug Shortages, Telehealth, & Biomarker Testing (2023).
[44]National Comprehensive Cancer Network Best Practices Committee, Carboplatin & Cisplatin Shortage Survey Results (June 7, 2023).
[45]A crash cart is a mobile unit that contains the materials, drugs, and devices necessary to address a variety of emergency or life-threatening situations.
[46]See GAO‑14‑194.
[47]Shortages were identified as critical because alternative drugs were not available, the shortages affected multiple manufacturers, or multiple shortage reports were received from different institutions.
[48]IQVIA Institute for Human Data Science, Drug Shortages in the U.S. 2023: A Closer Look at Volume and Price Dynamics (2023).
[49]Drug Shortage Staff document actions FDA takes to resolve a shortage, such as contact with manufacturers to increase drug supply, and the outcomes of these actions, among other information.
[50]FDA does not have guidance dictating what information Drug Shortage Staff must enter in Nexus, and the information entered into Nexus is at the discretion of the individual Drug Shortage Staff, according to one member of the Drug Shortage Staff. Due to this, the staff do not enter information into Nexus in a standardized or consistent way. For example, while some Drug Shortage Staff may document that they reached out to manufacturers others may include additional documentation, such as their emails with manufacturers.
[51]See GAO‑12‑116.
[52]See GAO‑14‑194.
[53]Two of members of the Drug Shortage Staff noted that FDA has changed its documentation system multiple times over recent years, with the most recent change being to Nexus, requiring them to learn a new system each time.
[54]See GAO‑14‑194.
[55]Pub. L. No. 116-136, § 3112(e), 134 Stat. 281, 363 (codified at 21 U.S.C. 360(j)(3)). Under this section, producers of drugs (registrants of drug establishments or their authorized agents) are required to submit reports on the amount of each listed drug manufactured, prepared, propagated, compounded, or processed for commercial distribution.
[56]See Food and Drug Administration, Reporting Amount of Listed Drugs and Biological Products Under Section 510(j)(3) of the FD&C Act Guidance for Industry (February 2024).
[57]See GAO‑21‑265.
[58]See GAO‑21‑265.
[59]In addition to the QMM program and risk management plans, FDA has other initiatives that could affect drug shortages. For example, we have previously reported on advanced manufacturing—innovative technologies that improve product quality and process performance—that FDA has highlighted as a way to enhance supply chain resiliency. For more information on advanced manufacturing, see GAO, Drug Manufacturing: FDA Should Fully Assess Its Efforts to Encourage Innovation, GAO‑23‑105650 (Washington, D.C.: Mar. 10, 2023).
[60]Food and Drug Administration, Drug Shortages: Root Causes and Potential Solutions (2019).
[61]All sites manufacturing a drug must adhere to minimum manufacturing standards to legally market drugs in the U.S. See generally 21 C.F.R. part 210. Compliance with these minimum requirements, called current good manufacturing practice, helps assure proper design, monitoring, and controls for manufacturing processes and facilities. FDA uses inspections and other activities to monitor sites manufacturing for the U.S. market for compliance with these requirements. However, adherence to minimum manufacturing standards does not indicate, for example, that a manufacturer is investing in improvements to prevent supply disruptions. Mature quality management helps assure that quality issues will not keep the drug from being available to patients and consumers, according to FDA.
[62]Mature quality management practices that would be assessed in a QMM program may include management commitment to quality, business continuity, and continual improvement processes.
[63]Department of Health and Human Services, Office of the Secretary, Policy Considerations to Prevent Drug Shortages and Mitigate Supply Chain Vulnerabilities in the United States (Apr. 2, 2024).
[64]Manufacturers required to develop, maintain, and implement a risk management plan are those manufacturers of prescription drugs and active pharmaceutical ingredients included in prescription drugs that are life-supporting, life-sustaining, intended for use in the prevention or treatment of a debilitating disease or condition, or are critical to the public health during a public health emergency. Pub. L. No. 116-136, § 3112(b), 134 Stat. 281, 362 (codified at 21 U.S.C. 356c(j)).
[65]White House, Fact Sheet: President Biden Announces New Actions to Strengthen America’s Supply Chains, Lower Costs for Families, and Secure Key Sectors (Washington, D.C.: Nov. 27, 2023), https://bidenwhitehouse.archives.gov/briefing-room/statements-releases/2023/11/27/fact-sheet-president-biden-announces-new-actions-to-strengthen-americas-supply-chains-lower-costs-for-families-and-secure-key-sectors/.
[66]ASPE is the principal advisor to the Secretary of Health and Human Services on policy development, and is responsible for major activities in policy coordination, legislation development, strategic planning, policy research, evaluation, and economic analysis.
[67]ASPE established the HHS Supply Chain Workgroup, including representatives from FDA, ASPR, and 10 other relevant agencies and offices, to guide the Coordinator’s activities. As of December 2024, the Workgroup had met twice. The Workgroup was tasked with developing a 4-year action plan that documented coordinated actions HHS planned; ASPE published the draft action plan on January 16, 2025.
[68]See Food and Drug Administration, Drug Shortages: Root Causes and Potential Solutions; and Strategic Plan for Preventing and Mitigating Drug Shortages (Silver Spring, MD.: Oct. 2013). Also see U.S. Senate Committee on Homeland Security and Governmental Affairs, Short Supply: Health and National Security Risks of Drug Shortages (Washington D.C.: March 2023); and Duke Margolis Center for Health Policy, Advancing Federal Coordination to Address Drug Shortages (Washington D.C.: Sept. 7, 2023).
[69]See GAO‑23‑105520.
[70]Department of Health and Human Services, National Strategy for a Resilient Public Health Supply Chain (Washington, D.C.: July 2021). This strategy was developed in response to Executive Order 14001, which directed the Department of Defense, Department of Homeland Security, and HHS, among others, to develop a supply chain resilience strategy to include an approach to design, build, and sustain long-term capability in the U.S. to manufacture supplies for future pandemics and biological threats. Federal Register, A Sustainable Public Health Supply Chain, § 4, 86 Fed. Reg. 7219, 7220–21 (Jan. 26, 2021).


